Bioprocessing Solutions
Adopting novel technologies to improve monoclonal antibody processes
Dr. Nandu Deorkar

Risk mitigation and process debottlenecking in continuous biomanufacturing
Dr. Pranav Vengsarkar

A Changing Landscape for Biopharma
Dr. Ger Brophy

Understanding monoclonal antibody aggregate behaviour impacting chromatographic process efficiency
Courtney O’Dell & Suman McLinden

Downstream Process Intensification with Novel Chromatography Resins for Enhanced Monoclonal Antibody Purification
Jonathan Fura, Jungmin Oh, Joshua Sumoski
Understanding the critical roles of cGMP chemicals and single-use technologies in cell & gene therapy manufacturing
Wayne Lynch & Timothy Korwan
Novel single-use solutions for cell & gene therapy manufacturing
Timothy Korwan & Jungmin Oh
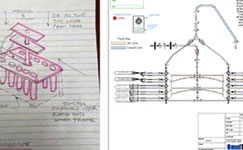
Designing Novel Fluid Sampling Systems for GMP Operations
Timothy Korwan, Tat Yuen