Western blotting antibody selection and detection methods
Introduction to western blotting
Western blotting is one of the most accurate protein detection methods used in countless medical and research applications. This complex laboratory technique when paired with the right tools and techniques can save your team’s time and cut costs.
In this article, we will dive into the details of antibody selection, detection methods, as well as outline the pros and cons of western blotting for protein detection.
Primary and secondary antibodies
Antibody bindings are critical in the binding and detection of proteins in western blotting. The primary antibodies are introduced in the beginning of the process to bind to the protein of interest. The primary antibody can sometimes be conjugated to a dye or enzyme for the detection phase, however if this is not added, a secondary antibody is necessary. The secondary antibodies are used to detect the primary antibody in the media. The two antibodies should be against the host species, meaning if the primary is mouse monoclonal, the secondary will need to be anti-mouse respectively. Using a secondary antibody instead of an enzyme or dye detection method opens up the opportunity for saving time and resources. The use of primary and secondary antibodies allows for the process of blot stripping.

There are three main advantages to blot stripping. This technique removes the primary and secondary antibodies from the blot that were used in detecting a protein. Blot stripping enables the blot to be re-probed to allow for detection of additional proteins on the same blot sample.
Additionally, blot stripping can be used to detect a loading control on the same blot. This negates user error or bias in detecting the levels of the target protein within the sample.
Lastly, blot stripping creates greater efficiency in blot technique, since the same sample can be reused. The ability to reuse samples aids in optimizing antibody concentration without wasting time or materials running multiple gel runs and transfers.
There are some considerations to make when setting up a blot stripping protocol. Stripping methods require a combination of detergent, reducing agent, heat, or low pH buffers to weaken the antigen-antibody interactions. A successful blot stripping should wash away the antibodies, while avoiding interference with the protein-membrane interactions.
Choice of membrane, detection method, stripping method, and sequence of protein detection must be compatible. Stripping is only successful when using detection methods like electrochemiluminescence (ECL) or fluorescent methods, unlike colorimetric methods that leave visible stains on the membrane. It is recommended to prioritize the use of low-affinity antibodies to detect low abundance proteins. This is due to the fact every additional stripping on a single gel some antigen loss is inevitable.
When reusing a blot, it’s important to thoroughly wash the stripped membrane before reuse, and then re-block to prevent non-specific binding of antibodies to the membrane.
Detection methods
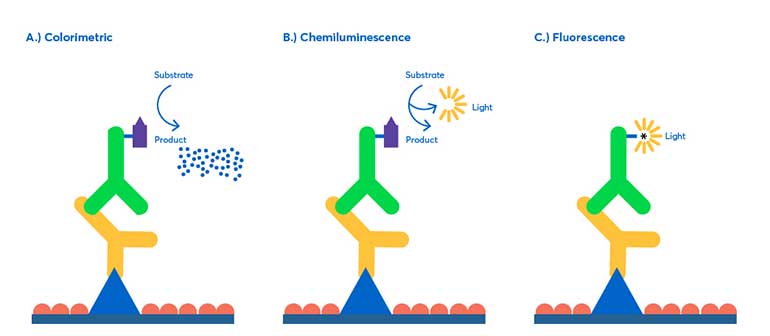
Detection on a western blot is the technique that makes visualizing the results possible. Detection methods work in a few ways; by an enzymatic reaction, or by conjugated antibodies that can be visualized directly.
Colorimetric technique
Colorimetric works by producing a colored precipitate via an enzymatic reaction. This technique is generally easier to use and does not require expensive materials such as x-ray film or a developer. Colorimetric has a lower picogram range than other techniques, and thus offers a range of sensitivity around 5-500 pg of protein, with a single output signal.
Chemiluminescence
Chemiluminescence is a highly sensitive detection method, more commonly used than colorimetric methods. Light is produced as a byproduct from a reaction between a chemical substrate and an enzyme. The light’s intensity is then measured by exposure to an X-ray film or charged coupled device (CCD) converting photons to an electronic signal.
There are two methods of Chemiluminescence with their own respective pros and cons. Chemiluminescence via film detection is a highly sensitive method detecting proteins within the femtogram range. It is a more expensive method, requiring film, developer maintenance, and a dark room. X-ray/CCD is typically easier to use and is more linear than film, but often requires more expensive equipment and extensive training.
Fluorescence
Fluorescence is the most widely used detection method to date in western blotting. Rather than relying on an enzymatic reaction to produce a signal, antibodies are conjugated to a specific fluorophore, which can be analyzed using an imaging system.
This allows for a far greater range of linearity than the previous methods of detection. Fluorescence allows for the probing of multiple proteins at once, with the use of various fluorophores. This can save considerable time being able to probe for proteins that run at a similar molecular weight.
Currently there are several imaging systems to choose from for detecting results for fluorescent western blotting, such as visible light, LED, or infrared lasers.
Electron transfer systems
Beyond tank and semi-dry transfer methods, there are a large variety of transfer methods to be used within these systems. Today, the most popular methodology is electroblotting, or electro transfer. This fast and effective transfer method can be used on wet, semi-dry, and dry transfer systems.
An electron transfer system is set up with the gel in direct contact with a piece of membrane formulated for protein-binding. These two layers are then placed between two electrodes, which are either submerged into buffer (tank systems) or encased with buffer saturated filter papers (dry and semi-dry systems).
When electroblotting, an electric field is applied, causing the proteins to move from the gel to the surface of the membrane, where they become bonded to the media.
Tank transfer (wet)
Wet transfers are the traditional method of blotting, requiring a longer window of transfer time of 30-120 minutes. The use of a buffer is critical for this technique, often requiring a methanol solution. This calls for more extensive cleaning protocols and disposal of hazardous materials.
Semi-dry
Semi-dry transfers are significantly faster than a standard tank transfer, requiring about 7-10 minutes. Buffers are still needed, but do not require any methanol to operate properly. This highly versatile technique makes for a slightly easier process, with less downtime and clean up. One of the greatest advantages to semi-dry transfer is that multiple methods can be used, creating opportunity for protocol customization.
Dry electroblotting
Dry transfer does not require any buffer solution and offers the fastest transfer time of 5-7 minutes total. This method utilizes a gel matrix that incorporates a buffer, rather than tanks or soaked filter papers. Dry transfer systems are incredibly easy to use, with high performance transfers, and minimal cleanup requirements compared to the other methods of transfer.
Advantage & disadvantages of electron transfer
Sensitivity & Accuracy
Western blotting technique offers the ability to detect proteins as small as 0.1 nanograms within a sample, making it an ideal tool for early diagnostics. This heightened sensitivity also cuts down the need for antibodies, requiring fewer for testing and significantly cutting down on laboratory costs.
Western blot is also dependably precise for protein detection. This is because the electrophoresis sorts proteins from your sample by size, charge, and conformation. Specificity is also supported by the antibody-antigen interaction, which aids in the detection of a target protein in a complex mixture. This can be done with over 300,000 different proteins present in a given sample.
Subjective results & costly demands
Although western blot can provide precise results, it can also produce misleading results if there are errors in your technique. False-positives can occur due to an antibody reacting with a non-intended protein, such as with parasitic infections. Whereas a false-negative can happen when larger proteins are not give sufficient transfer time.
When blotting techniques are inaccurate, they often produce faded, skewed bands that are subject to interpretation by a lab technician. It is important to have control tests and to understand each step in the western blot process in order to get clear and dependable results.
Proper training and laboratory equipment are needed to successfully blot. Understanding reagent concentrations, incubation times, and detection protocols require extensive training and should be performed by skilled laboratory staff.
Conclusion
Whether you are looking for transfer membranes, blotting kits, specified reagents, transfer systems, or unique antibodies, Avantor has the solutions for your laboratory needs. Through our website, you can find extensive services and resources to help along the way.