Clinical Trials Director
Simplify Your Clinical Trials
In the past decade, the total number of registered clinical trials has grown by almost 400%1, with more than a quarter of all current studies taking place outside of North America and Europe2. This global growth matched with constant innovation in testing methods and therapies makes your work dynamic, if not difficult.
You’re now concerned with more than just managing multiple CROs, vendors, project managers, and ever-changing trial needs. You have to balance complex international and regional regulations with product availability and timely delivery, all while maintaining a visible and traceable chain of custody, from start to finish.
Your challenges are clear, but are your solutions? Three strategic acquisitions by VWR provide a platform to help you meet these emerging challenges. Therapak, MESM, and EPL Archives each offer industry-leading solutions developed specifically for clinical trial studies. From custom clinical kits, to equipment and ancillaries management, and biorepository services, we can help you address the needs of a rapidly changing clinical trial any where in the world. Join us and find your solutions below.
Service Solution Spotlight
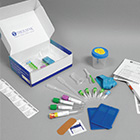
Custom Specimen Collection Kits
Find out how Avantor Services provided a clinical lab with kits designed to retrieve specific biological specimens from the field and ship directly to labs for analysis.
Find Helpful Resources
Explore Our Holistic Solution for Simplifying Clinical Trial Management

Custom Clinical Kits and Supplies
Therapak supplies pre-packaged convenience kits, procedure packs, and customized kits for the clinical trial, pharmaceutical, diagnostic, and clinical laboratory.
Clinical Equipment and Ancillaries Management
MESM is a well-known, highly respected, and ISO 9001-certified provider of equipment and ancillary supplies for clinical trials in over 80 countries.

Archive and Biorepository Services
EPL Archives is an international biorepository services organization that supports customers across the entire regulated product research, development, and commercialization lifecycle.

Histology Services
Learn how our team of certified histologists can accelerate the production of slides critical to new drug discovery.

Chemical Management
Outsourcing delivers comprehensive solutions to chemical management challenges

Instrument Calibration & Validation
Keep your results accurate and consistent with these calibration services, ensuring the right reading every time.
Take the Next Step

1 https://clinicaltrials.gov/ct2/resources/trends
2 https://clinicaltrials.gov/ct2/search/map